Acidifying Oceans: Oceans and Climate Change

This short video draws together ocean acidification and its anthropogenic causes.
Leave an egg in a cup of vinegar overnight, and the shell will start to dissolve off the egg.
This is because the acetic acid in the vinegar neutralizes the calcium carbonate in the eggshell, releasing carbon dioxide and weakening the shell. Now imagine this same science experiment but with an ocean full shellfish in a sea of acidifying water (less basic, to be accurate). With current carbon dioxide (CO2) emissions levels, we are running this experiment in real-time. The ocean acts as a carbon sink, dissolving about 30 percent of CO2 released into the atmosphere. The CO2 in the ocean decreases the pH of the seawater, which can have a similar “dissolving” effect on calcifying organisms—animals that have hard structures such as corals, oysters, and sea urchins. Ocean acidification also affects the ecosystem as a whole by disrupting food webs.
Download an SOS playlist for this module.

This short video draws together ocean acidification and its anthropogenic causes.

A short film that explores the impacts of climate change on our oceans, including its impacts on ecosystems.
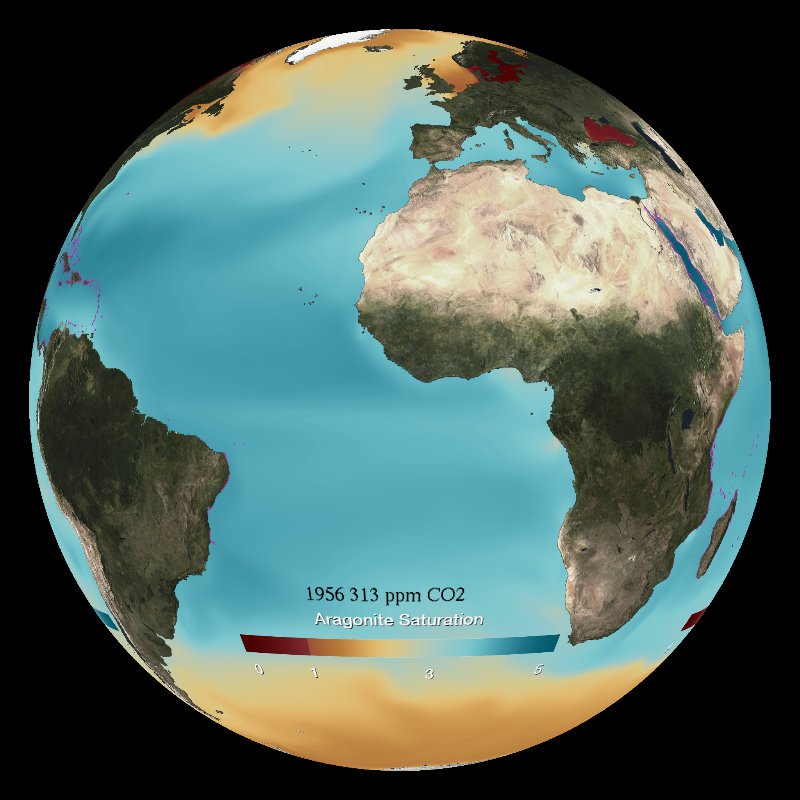
These two datasets model the past and project the future aragonite saturation and surface pH from 1861 to 2100. The factors measured are both indicators of ocean acidification.
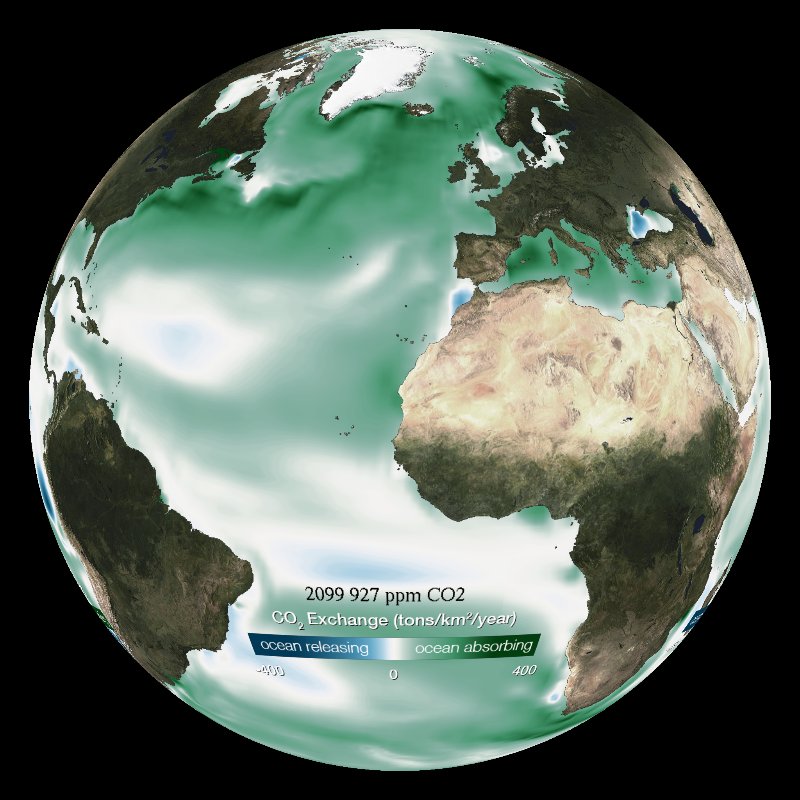
The ocean serves as a carbon sink, however as it absorbs more and more CO2 (as seen in this model), it becomes less basic than before.
For SOSx use Carbon Dioxide Concentration: GEOS-5 Model.